Global Artificial Pancreas Device System (APDS) Market, By Type (Threshold Suspended Device System, CTR System, CTT System), By Application, and By Region - Trends and Forecast Analysis, 2021-2035
Publish Date: 2025-05-01 | Format: PDF | Category: Healthcare and Pharmaceutical | Pages: 390
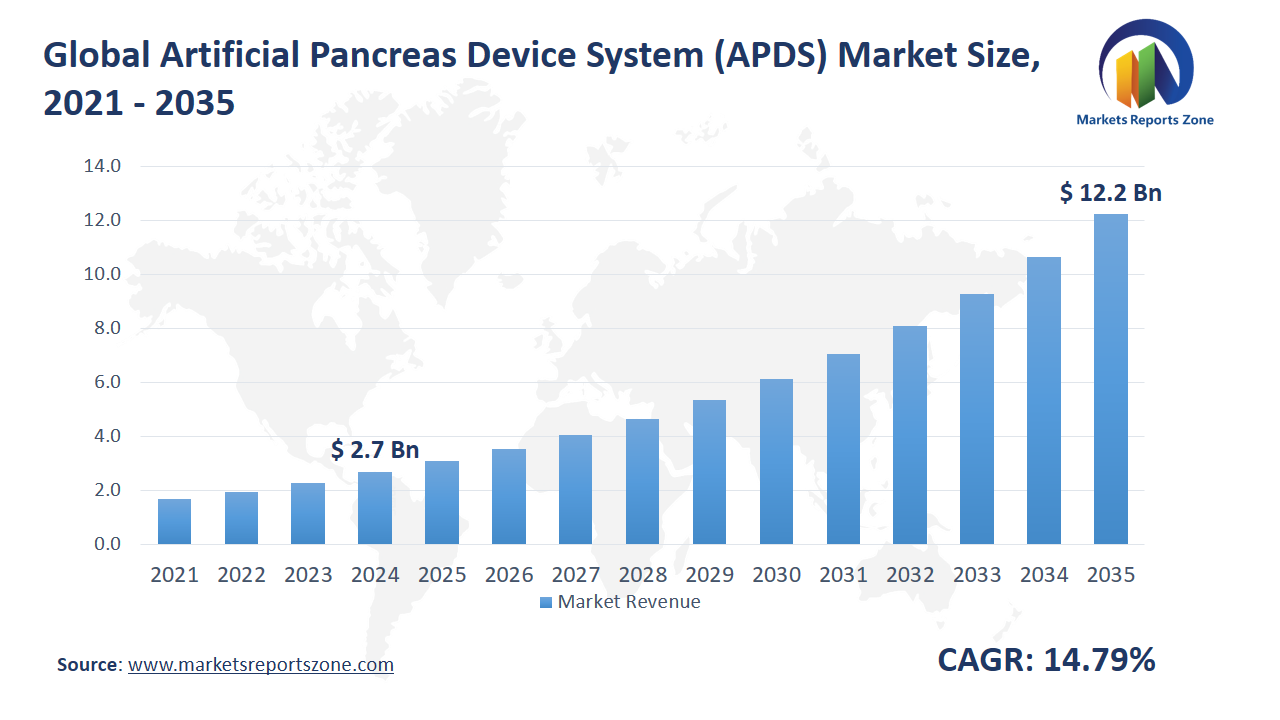
Global Artificial Pancreas Device System (APDS) Market Size is expected to reach USD 12.25 Billion by 2035 from USD 2.68 Billion in 2024, with a CAGR of around 14.79% between 2024 and 2035. The global Artificial Pancreas Device System (APDS) market is being propelled by two primary drivers: the increasing prevalence of diabetes and technological advancements in diabetes management. The rising number of diabetes cases worldwide has heightened the demand for effective glucose control solutions. Technological innovations, such as continuous glucose monitoring (CGM) and automated insulin delivery systems, have enhanced the efficacy of APDS, making them more appealing to both patients and healthcare providers. For instance, the NHS in England has initiated the distribution of artificial pancreas devices to thousands of individuals with type 1 diabetes, aiming to eliminate the need for daily insulin injections.
However, the high cost associated with APDS remains a significant restraint, limiting accessibility for many patients. Despite this, opportunities exist in the integration of advanced technologies and expanding market penetration in emerging economies. The incorporation of artificial intelligence and machine learning into APDS can further enhance their functionality, offering personalized diabetes management solutions. Additionally, emerging markets present significant growth prospects due to increasing healthcare awareness and infrastructure development. For example, the FDA's approval of the CamAPS FX artificial pancreas system for use during pregnancy marks a significant advancement in personalized diabetes care.
Driver: Rising Diabetes Cases Fuel Demand
A steady increase in diabetes cases has significantly driven the global demand for Artificial Pancreas Device Systems. This trend has been noticed especially in urban populations where sedentary lifestyles and poor dietary habits are more common. With more people being diagnosed at a younger age, long-term glucose management has become a top priority. Artificial pancreas systems, designed to automate insulin delivery, have been adopted to reduce manual intervention and improve health outcomes. In many developed countries, patients have shifted from traditional insulin injections to closed-loop systems that mimic natural pancreatic function. For example, a 16-year-old athlete in California was able to maintain stable glucose levels during high-intensity training after switching to an artificial pancreas. In India, a working professional struggling with erratic sugar spikes found relief using a hybrid closed-loop system. Parents of children with type 1 diabetes have increasingly turned to these systems for peace of mind, especially overnight when glucose monitoring becomes more difficult. The rising awareness about early diabetes control and the growing preference for automated devices have boosted acceptance. As the burden of diabetes grows globally, this driver will continue to shape the adoption of advanced and intelligent diabetes management technologies.
Key Insights:
- The adoption rate of artificial pancreas device systems among U.S. Type 1 diabetes patients is estimated at about 15% in 2024.
- In Germany, approximately 9% of adults with diabetes are potential candidates for APDS, reflecting growing penetration in Europe.
- The U.S. Medicare and Medicaid programs now reimburse APDS for eligible patients, increasing accessibility and adoption.
- Major companies have invested over $500 million in R&D for APDS technology development and clinical trials in the past five years.
- In 2025, threshold suspend device systems are projected to account for 72.5% of the global APDS product segment.
- The number of APDS units sold globally surpassed 100,000 in 2024, with rapid growth in North America and Europe.
- The penetration rate of APDS in U.S. hospitals and diabetes clinics is estimated at 22% as of 2024.
Segment Analysis:
The Artificial Pancreas Device System (APDS) market is segmented by type into Threshold Suspended Device Systems, Control-to-Range (CTR) Systems, and Control-to-Target (CTT) Systems, each offering different levels of glucose regulation. Threshold Suspended Device Systems are designed to stop insulin delivery when glucose levels fall too low, reducing the risk of hypoglycemia. These systems are often used in children or elderly patients who are prone to sudden glucose drops. CTR Systems aim to maintain glucose within a safe range by adjusting insulin levels periodically, and are widely used by active adults managing fluctuating sugar levels due to inconsistent routines. CTT Systems, being the most advanced, automatically aim for and maintain a specific glucose target. These are preferred by patients with type 1 diabetes seeking tighter control and fewer interventions. In terms of application, hospitals primarily use these systems for inpatient diabetes management, especially during surgeries or post-operative recovery. For instance, patients recovering from cardiac procedures are monitored using CTR systems to avoid glycemic complications. Clinics use APDS for long-term outpatient care, helping patients transition from manual insulin injections to automated solutions. A diabetic schoolteacher in France recently adopted a CTT system through her local clinic and reported improved glucose stability and reduced stress during work hours.
Regional Analysis:
The global Artificial Pancreas Device System (APDS) market has seen varied growth across regions, shaped by healthcare infrastructure, awareness, and regulatory support. In North America, adoption has been widespread due to strong insurance coverage and patient education. A college student in Canada with type 1 diabetes recently benefited from a government-supported program that provided access to a hybrid closed-loop system, improving his quality of life during exams. In Europe, innovation and public health initiatives have driven usage. A retiree in Sweden, previously relying on manual insulin doses, now manages his condition with a Control-to-Target device, experiencing fewer hypoglycemic episodes. Asia-Pacific has witnessed rising demand, mainly due to growing urban populations and increasing diabetes cases. A working mother in Japan now uses a threshold suspend system provided through a corporate wellness plan, allowing her to better manage her condition while juggling work and childcare. In Latin America, access remains limited but improving, with clinics in Brazil recently introducing APDS technology through pilot programs. In the Middle East and Africa, growth has been slower, but interest is growing. A teenager in the UAE recently became one of the first in her community to start APDS therapy through a private hospital, showing promising early results.
Competitive Scenario:
The Artificial Pancreas Device System (APDS) market has been rapidly advancing, with key companies driving innovation and accessibility through recent developments. Medtronic continues refining its hybrid closed-loop systems, focusing on improved user experience for both children and adults. Bigfoot Biomedical has shifted its strategy by licensing insulin pump patents to Insulet, allowing it to focus more on smart injection technologies like the Bigfoot Unity system. Johnson & Johnson, though quieter in recent years, has contributed to the foundational technology behind automated insulin delivery. Tandem Diabetes Care has expanded its partnerships, integrating the Dexcom G7 CGM into its insulin pumps, aiming for tighter glucose control with seamless interoperability. Pancreum Inc. remains in the development phase with its modular AP system, intended to offer users customizable insulin therapy. TypeZero Technologies, known for its algorithms, is collaborating with Tandem to accelerate the rollout of more responsive closed-loop systems. Beta Bionics has made a major leap with its iLet Bionic Pancreas receiving regulatory clearance; the device determines all insulin doses autonomously based only on body weight. These companies are reshaping diabetes care through automation, convenience, and precision, offering patients—from school-aged children to working adults—better glycemic control and reduced burden in daily disease management.
Artificial Pancreas Device System (APDS) Market Report Scope
| Report Attribute | Details |
|---|---|
| Market Size Value in 2024 | USD 2.68 Billion |
| Revenue Forecast in 2035 | USD 12.25 Billion |
| Growth Rate | CAGR of 14.79% from 2025 to 2035 |
| Historic Period | 2021 - 2024 |
| Forecasted Period | 2025 - 2035 |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Regions Covered | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
| Countries Covered | U.S.; Canada; Mexico, UK; Germany; France; Spain; Italy; Russia; China; Japan; India; South Korea; Australia; Southeast Asia; Brazil; Argentina; Saudi Arabia; UAE; South Africa |
| Key companies profiled | Medtronic; Bigfoot Biomedical; Johnson & Johnson; Tandem Diabetes Care Inc; Pancreum Inc; TypeZero Technologies, LLC; Beta Bionics |
| Customization | Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
The Global Artificial Pancreas Device System (APDS) Market report is segmented as follows:
By Type,
- Threshold Suspended Device System
- CTR System
- CTT System
By Application,
- Hospitals
- Clinics
By Region,
- North America
- U.S.
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Spain
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Rest of Middle East and Africa
Key Market Players,
- Medtronic
- Bigfoot Biomedical
- Johnson & Johnson
- Tandem Diabetes Care Inc
- Pancreum Inc
- TypeZero Technologies, LLC
- Beta Bionics
Frequently Asked Questions
Research Objectives
- Proliferation and maturation of trade in the global Artificial Pancreas Device System (APDS) Market.
- The market share of the global Artificial Pancreas Device System (APDS) Market, supply and demand ratio, growth revenue, supply chain analysis, and business overview.
- Current and future market trends that are influencing the growth opportunities and growth rate of the global Artificial Pancreas Device System (APDS) Market.
- Feasibility study, new market insights, company profiles, investment return, market size of the global Artificial Pancreas Device System (APDS) Market.
Chapter 1 Artificial Pancreas Device System (APDS) Market Executive Summary
- 1.1 Artificial Pancreas Device System (APDS) Market Research Scope
- 1.2 Artificial Pancreas Device System (APDS) Market Estimates and Forecast (2021-2035)
- 1.2.1 Global Artificial Pancreas Device System (APDS) Market Value and Growth Rate (2021-2035)
- 1.2.2 Global Artificial Pancreas Device System (APDS) Market Price Trend (2021-2035)
- 1.3 Global Artificial Pancreas Device System (APDS) Market Value Comparison, by Type (2021-2035)
- 1.3.1 Threshold Suspended Device System
- 1.3.2 CTR System
- 1.3.3 CTT System
- 1.4 Global Artificial Pancreas Device System (APDS) Market Value Comparison, by Application (2021-2035)
- 1.4.1 Hospitals
- 1.4.2 Clinics
Chapter 2 Research Methodology
- 2.1 Introduction
- 2.2 Data Capture Sources
- 2.2.1 Primary Sources
- 2.2.2 Secondary Sources
- 2.3 Market Size Estimation
- 2.4 Market Forecast
- 2.5 Assumptions and Limitations
Chapter 3 Market Dynamics
- 3.1 Market Trends
- 3.2 Opportunities and Drivers
- 3.3 Challenges
- 3.4 Market Restraints
- 3.5 Porter's Five Forces Analysis
Chapter 4 Supply Chain Analysis and Marketing Channels
- 4.1 Artificial Pancreas Device System (APDS) Supply Chain Analysis
- 4.2 Marketing Channels
- 4.3 Artificial Pancreas Device System (APDS) Suppliers List
- 4.4 Artificial Pancreas Device System (APDS) Distributors List
- 4.5 Artificial Pancreas Device System (APDS) Customers
Chapter 5 COVID-19 & Russia?Ukraine War Impact Analysis
- 5.1 COVID-19 Impact Analysis on Artificial Pancreas Device System (APDS) Market
- 5.2 Russia-Ukraine War Impact Analysis on Artificial Pancreas Device System (APDS) Market
Chapter 6 Artificial Pancreas Device System (APDS) Market Estimate and Forecast by Region
- 6.1 Global Artificial Pancreas Device System (APDS) Market Value by Region: 2021 VS 2023 VS 2035
- 6.2 Global Artificial Pancreas Device System (APDS) Market Scenario by Region (2021-2023)
- 6.2.1 Global Artificial Pancreas Device System (APDS) Market Value Share by Region (2021-2023)
- 6.3 Global Artificial Pancreas Device System (APDS) Market Forecast by Region (2024-2035)
- 6.3.1 Global Artificial Pancreas Device System (APDS) Market Value Forecast by Region (2024-2035)
- 6.4 Geographic Market Analysis: Market Facts and Figures
- 6.4.1 North America Artificial Pancreas Device System (APDS) Market Estimates and Projections (2021-2035)
- 6.4.2 Europe Artificial Pancreas Device System (APDS) Market Estimates and Projections (2021-2035)
- 6.4.3 Asia Pacific Artificial Pancreas Device System (APDS) Market Estimates and Projections (2021-2035)
- 6.4.4 Latin America Artificial Pancreas Device System (APDS) Market Estimates and Projections (2021-2035)
- 6.4.5 Middle East & Africa Artificial Pancreas Device System (APDS) Market Estimates and Projections (2021-2035)
Chapter 7 Global Artificial Pancreas Device System (APDS) Competition Landscape by Players
- 7.1 Global Top Artificial Pancreas Device System (APDS) Players by Value (2021-2023)
- 7.2 Artificial Pancreas Device System (APDS) Headquarters and Sales Region by Company
- 7.3 Company Recent Developments, Mergers & Acquisitions, and Expansion Plans
Chapter 8 Global Artificial Pancreas Device System (APDS) Market, by Type
- 8.1 Global Artificial Pancreas Device System (APDS) Market Value, by Type (2021-2035)
- 8.1.1 Threshold Suspended Device System
- 8.1.2 CTR System
- 8.1.3 CTT System
Chapter 9 Global Artificial Pancreas Device System (APDS) Market, by Application
- 9.1 Global Artificial Pancreas Device System (APDS) Market Value, by Application (2021-2035)
- 9.1.1 Hospitals
- 9.1.2 Clinics
Chapter 10 North America Artificial Pancreas Device System (APDS) Market
- 10.1 Overview
- 10.2 North America Artificial Pancreas Device System (APDS) Market Value, by Country (2021-2035)
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.2.3 Mexico
- 10.3 North America Artificial Pancreas Device System (APDS) Market Value, by Type (2021-2035)
- 10.3.1 Threshold Suspended Device System
- 10.3.2 CTR System
- 10.3.3 CTT System
- 10.4 North America Artificial Pancreas Device System (APDS) Market Value, by Application (2021-2035)
- 10.4.1 Hospitals
- 10.4.2 Clinics
Chapter 11 Europe Artificial Pancreas Device System (APDS) Market
- 11.1 Overview
- 11.2 Europe Artificial Pancreas Device System (APDS) Market Value, by Country (2021-2035)
- 11.2.1 UK
- 11.2.2 Germany
- 11.2.3 France
- 11.2.4 Spain
- 11.2.5 Italy
- 11.2.6 Russia
- 11.2.7 Rest of Europe
- 11.3 Europe Artificial Pancreas Device System (APDS) Market Value, by Type (2021-2035)
- 11.3.1 Threshold Suspended Device System
- 11.3.2 CTR System
- 11.3.3 CTT System
- 11.4 Europe Artificial Pancreas Device System (APDS) Market Value, by Application (2021-2035)
- 11.4.1 Hospitals
- 11.4.2 Clinics
Chapter 12 Asia Pacific Artificial Pancreas Device System (APDS) Market
- 12.1 Overview
- 12.2 Asia Pacific Artificial Pancreas Device System (APDS) Market Value, by Country (2021-2035)
- 12.2.1 China
- 12.2.2 Japan
- 12.2.3 India
- 12.2.4 South Korea
- 12.2.5 Australia
- 12.2.6 Southeast Asia
- 12.2.7 Rest of Asia Pacific
- 12.3 Asia Pacific Artificial Pancreas Device System (APDS) Market Value, by Type (2021-2035)
- 12.3.1 Threshold Suspended Device System
- 12.3.2 CTR System
- 12.3.3 CTT System
- 12.4 Asia Pacific Artificial Pancreas Device System (APDS) Market Value, by Application (2021-2035)
- 12.4.1 Hospitals
- 12.4.2 Clinics
Chapter 13 Latin America Artificial Pancreas Device System (APDS) Market
- 13.1 Overview
- 13.2 Latin America Artificial Pancreas Device System (APDS) Market Value, by Country (2021-2035)
- 13.2.1 Brazil
- 13.2.2 Argentina
- 13.2.3 Rest of Latin America
- 13.3 Latin America Artificial Pancreas Device System (APDS) Market Value, by Type (2021-2035)
- 13.3.1 Threshold Suspended Device System
- 13.3.2 CTR System
- 13.3.3 CTT System
- 13.4 Latin America Artificial Pancreas Device System (APDS) Market Value, by Application (2021-2035)
- 13.4.1 Hospitals
- 13.4.2 Clinics
Chapter 14 Middle East & Africa Artificial Pancreas Device System (APDS) Market
- 14.1 Overview
- 14.2 Middle East & Africa Artificial Pancreas Device System (APDS) Market Value, by Country (2021-2035)
- 14.2.1 Saudi Arabia
- 14.2.2 UAE
- 14.2.3 South Africa
- 14.2.4 Rest of Middle East & Africa
- 14.3 Middle East & Africa Artificial Pancreas Device System (APDS) Market Value, by Type (2021-2035)
- 14.3.1 Threshold Suspended Device System
- 14.3.2 CTR System
- 14.3.3 CTT System
- 14.4 Middle East & Africa Artificial Pancreas Device System (APDS) Market Value, by Application (2021-2035)
- 14.4.1 Hospitals
- 14.4.2 Clinics
Chapter 15 Company Profiles and Market Share Analysis: (Business Overview, Market Share Analysis, Products/Services Offered, Recent Developments)
- 15.1 Medtronic
- 15.2 Bigfoot Biomedical
- 15.3 Johnson & Johnson
- 15.4 Tandem Diabetes Care Inc
- 15.5 Pancreum Inc
- 15.6 TypeZero Technologies, LLC
- 15.7 Beta Bionics
Report ID:
207
Published Date:
May 2025
Trusted by more than 10,500 organizations globally



Infaluble Methodology
Customization
Analyst Support
Targeted Market View